Artículos
Salt stress in melon (Cucumis melo L.) is alleviated by seed treatment with melatonin, modifying physiological and biochemical parameters
El estrés salino en melón (Cucumis melo L.) es aliviado parcialmente con el tratamiento de semillas con melatonina, modificando parámetros fisiológicos y bioquímicos
Revista FAVE Sección Ciencias Agrarias
Universidad Nacional del Litoral, Argentina
ISSN: 2346-9129
ISSN-e: 2346-9129
Periodicity: Semestral
no. 23, e0027, 2024
Received: 21 December 2023
Accepted: 13 March 2024

This work is licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International.
Abstract: Melatonin (N-acetyl-5-methoxytryptamine) is a molecule with a reported importance in increasing tolerance to several types of stress. The present study investigates the effect of melon (Cucumis melo L.) seed treatment with melatonin solutions (0, 10, 50 and 100 μM) at two durations (6 and 12 h) on germination and initial growth of melon plants in salt stress. Germination under salt stress (14 dS m-1 EC) suffered a decrease, which is reversed after seed treatment with melatonin and followed to 80% with 10 and 50 μM solutions. Then, considering plant growing in salt stress (8 dS m-1 EC), the best growth and physiological and biochemical responses (xylem water potential, leaf relative water content, total chlorophyll, root viability, proline, malondialdehyde content, peroxidase and catalase activity) were measured in plants derived from 50 μM seed treatment. No changes were observed at the anatomical level. Results suggest that melatonin can alleviate the effect of salt stress during seed germination and early plant growth. There is a dose-dependent response.
Keywords: early growth, exogenous melatonin, germination, reactive oxygen species, salt stress.
Resumen: La melatonina (N-acetil-5-metoxitriptamina) es una molécula con una reportada importancia en el incremento de la tolerancia a varios tipos de estrés. El presente estudio investiga el efecto del tratamiento de semillas de melón (Cucumis melo L.) con soluciones de melatonina (0, 10, 50 y 100 μM) en dos duraciones (6 y 12 h) sobre la germinación y el crecimiento inicial de plantas de melón en estrés salino. La germinación bajo estrés salino (14 dS m-1 CE) experimentó una disminución, la cual se revirtió luego del tratamiento de las semillas con melatonina, llegando al 80% con soluciones 10 y 50 μM. Luego, considerando las plantas creciendo en estrés salino (8 dS m-1 CE), se obtuvieron las mejores respuestas de crecimiento y fisiológicas y bioquímicas (potencial hídrico del xilema, contenido hídrico relativo de las hojas, clorofila total, viabilidad de las raíces, prolina, contenido de malondialdehído, actividad peroxidasa y catalasa) en plantas provenientes del tratamiento de semillas con 50 μM. No se observaron cambios a nivel anatómico. Los resultados sugieren que la melatonina puede aliviar el efecto del estrés salino durante la germinación de las semillas y el crecimiento temprano de las plantas. Hay una respuesta dosis dependiente.
Palabras clave: crecimiento inicial, melatonina exógena, germinación, especies reactivas al oxígeno, estrés salino.
Introduction
Soil salinity is one of the limitations that most affect world crop production (Corwin, 2021), particularly severe in irrigated soils. It has been estimated that more than 20% , 45 million ha of irrigated land, that represents near to 10% of world’s food production, is affected by salinity, and this area continues to increase (Pandey et al., 2019). Salts in irrigation water come from natural weathering and dissolution of soil parent material and accumulate as water evaporates or is consumed by crops (Yasuor et al. 2020).
As consequence of salinity all aspects of plant development are affected, due to a combination of osmotic stress and ionic toxicity (Hussain et al., 2019). High salt concentration in water reduces osmotic potential (Ψo) and consequently water movement and availability in the soil-plant system. So, cell expansion can be reduced or inhibited (Hailu & Mehari, 2021). This osmotic effect can also cause stomatal closure, that difficult the entry of CO., affecting indirectly photosynthesis (Shabala & Munns, 2017). Ionic toxicity, or ionic stress, is mainly due to the excess Na. and Cl. on critical biochemical processes in plant tissues (Yasuor, el at. 2020). A high level of Na. can interfere with the absorption of other cations such as K., Mg2+ and NH.. (Safdar et al., 2019). Membrane depolarization occurs when positively charged Na. crosses the plasma membrane, affecting uptake of many essential cations and increasing efflux of some of them (e.g. K.) (Shabala & Munns, 2017). Chloride is an essential micronutrient that regulates enzyme activities in the cytoplasm, co-factor in photosynthesis, but it can be toxic at high concentration (Geilfus, 2018). Salinity-stressed plants exhibit dramatically altered levels of many endogenous physiological signaling. Among these, the accumulation of reactive oxygen species (ROS) (Choudhury et al., 2017). These ROS can lead to oxidative damages in cellular components such as proteins, lipids, and DNA, and can lead to cell injury and death (Hasanuzzaman et al., 2021).
Worldwide melon production is 28 million Mgs, with a production area of 1 million ha (FAOSTAT, 2023). It is usually cultivated intensively and with complementary irrigation (Singh & Bharati, 2016). Salinity is one of the main factors limiting melon production (Sarabi et al., 2017). Although it is considered a moderately tolerant crop to salinity (Huang et al., 2012), the initial phases of germination, emergence and seedling, are highly significantly affected (Pinheiro et al., 2019). Some strategies to alleviate the detrimental effect of salinity in melon are grafting onto tolerant rootstocks (Ulas et al., 2019), proper irrigation management (Suárez-Hernández et al., 2019), pre-sowing seed treatments (Oliveira et al., 2019), plant growth-promoting bacteria (Gopalakrishnan et al., 2022) and exogenous application of specific compounds (e.g. nutrients, amino acids, plant growth regulators) (Jin et al., 2019; Shalaby & El-Messairy, 2018).
Melatonin (N-acetyl-5-methoxytryptamine) is a molecule isolated for the first time in the bovine pineal gland (Lerner et al., 1958). For a long time, it was considered a neurohormone exclusive to mammals (Nawaz et al., 2016), with important roles in regulation of circadian rhythm, antioxidant enzyme activity, seasonal reproductive physiology and aging (Wu et al. 2021; Fan et al. 2018). In 1995, two groups of researchers (Dubbels et al., 1995; Hattori et al., 1995), working separately, detected its presence in some edible plants and since then the number of publications reporting its existence in plants has been increasing (Wu et al. 2021; Arnao and Hernández-Ruiz 2019). The main functions are associated with its antioxidant capacity (Sharif et al., 2018) by stimulating the activity of antioxidant enzymes or directly neutralizing reactive oxygen species (ROS) (Zhang and Zhang 2014). In addition, a growing number of studies have shown the advantages of melatonin application, for the improvement of adaptation and survival against different types of stress (Debnath et al., 2019). A specific melatonin receptor (CAND2/PMTR1) was discovered in Arabidopsis thaliana (Wei et al. 2018). This receptor is localized in the plasma membrane and interacts with the receptor-dependent stomatal closure (GPA1) via an NADPH oxidase-mediated ROS signaling pathway (Arnao & Hernández-Ruiz, 2020).
Previously, Castañares and Bouzo (2019) determined that melatonin applied to cantaloupe melon seeds can alleviate the effects of salt stress on germination and the initial growth of plants grown in saline conditions. In a recent work (Liu et al., 2020) the authors explained part of the mode of action of melatonin, although these experiments were carried out on rice. They evaluated the downstream components of melatonin signaling that lead to increased salinity stress tolerance, at the cellular and molecular levels. Eisa et al. (2023) analyzed the efficacy of melatonin in enhancing the salinity stress tolerance, but using an ornamental plant, the persian buttercup (Ranunculus asiaticus L.). These authors revealed the significant effectiveness of exogenous melatonin treatments for enhancing different traits of plant growth. Although there is a relatively abundant bibliography on the effect of melatonin in plants, it is very scarce in melon. In the work carried out here, the purpose was to investigate some of the possible physiological mechanisms that operate in melon under conditions of saline stress, in response to imbibition with melatonin.
Materials and methods
Plant material
Honeydew melon (Cucumis melo L.) seeds `Green Flesh´ variety, which are widely cultivated in Argentina, were used in the present work. Melatonin (N-acetyl-5-methoxytryptamine) was purchased from Droguería Saporiti (Buenos Aires, Argentina). All reagents and salts (analytical grade) used in the experiments were obtained from Sigma-Aldrich (Buenos Aires, Argentina).
Effect of seed treatment with melatonin on germination of melon seeds in salinity stress
Seeds were surface sterilized in a 10% sodium hypochlorite solution during 5 min, washed with deionized water, placed between two filter papers (Whatman No. 1) in 8.5 cm Petri dishes and soaked for 6 or 12 h in solutions containing 0, 10, 50 and 100 μM melatonin respectively, in a ratio 1:5 (weight: volume). The assay was performed in a germination chamber (Semedic I-500 PF, Argentina) at 25 °C (± 0.5 °C) and dark. After treatment the seeds were removed, rinsed three times in deionized water and air-dried at 25 °C for 24 h to reduce moisture content to <10 %. Later, seeds were put to germinate between two filter papers (Whatman No. 1) in 8.5 cm Petri dishes and soaked with 140 mM NaCl solution (Electrical conductivity, EC: 14 dS m-1) in a germination chamber at 25 °C (± 0.5 °C) in dark. Control treatment (Control S0) consisted in seeds soaked with distilled water (0 dS m-1). After 8 days the number of germinated seeds was recorded (ISTA, 2022). Seeds were considered germinated when the radicle was visible. Germination percentage (GP) was calculated according to the following formula: GP (%) = (n/N) × 100, where n is the number of seeds germinated during the experiment and N is the total number of seeds (ISTA 2022). Five replicates per treatment with ten seeds per replication were used.
Effect of seed treatment with melatonin on initial growth of melon plants in salinity stress
Seeds were treated as 2.2 with 0, 10, 50 and 100 μM melatonin solution and the time of imbibition that allowed the best germination in previous experiment. After treatment seeds were rinsed three times in distilled water and sown in 1000 cm3 pots filled with perlite, and watered with Hoagland nutrient solution (Hoagland & Arnon, 1950) with 2.0 dS m-1 EC.
After first leaf emergence, plants started to be watered with the nutrient solution and the addition of approximately 60 mM NaCl, to obtain 8.0 dS m-1 EC. The EC value was monitored daily using a portable conductivity meter (Hanna model HI98304, Italy). Control treatment (Control S0) consisted in plants derived from non-treated seeds and watered only with Hoagland nutrient solution. The experiment was performed in a phytotron under the following conditions: 60% RH, 16 h light length, 400 μmol-2s-1 light intensity and day/night temperatures of 25/18 °C.
The experiment had a completely randomized design with 40 replications per treatment. Data obtained were analyzed using Tukey’s test at 0.05 confidence level using Infostat statistical software (Di Rienzo et al., 2011).
-Growth evaluation
Five plants were extracted from each treatment after 40 days to determinate the following phytometric parameters: total dry weight, main stem length, number of leaves, and leaf area. Total dry weight was obtained by introducing the samples in an oven at 60 °C until constant weight was attained (Miller, 1997). Stem length was obtained by measuring the extension between the insertion of the cotyledons and the apical end. Leaves number was determined out by counting, considering only those that presented their expanded leaf blade. Thus, the very small leaflets surrounding the terminal bud were not considered. Leaf area per plant was measured using Image J image processing software (Schneider et al. 2012).
To analyze the global effects of salinity and each treatment on growth, a Biometric Parameters Index (BPI) was used, according to the following expression (Larraburu & Llorente, 2015):
BPI (𝑡) =∑4 (𝑉𝑝 (𝑖, 𝑡) − 𝑋(𝑖))/𝐷𝑆(𝑖)
𝑖=1
Where:
𝑉𝑝 (𝑖, 𝑡) = Value for the phytometric parameter 𝑖 in treatment 𝑡
𝑋(𝑖) = Average of the phytometric parameter 𝑖
𝐷(𝑖) = Standard deviation for the phytometric parameter 𝑖
-Xylem water potential measurement
After 20 days of growth, xylem water potential (Ψx) of plants was measured with a pressure chamber a Schölander-type (Model 0-6 MPA, BioControl, Argentina). Measurements were made in situ on developed leaves, located in the middle third of the stem (Shabala et al., 2010).
-Determination of leaf relative water content
Leaf relative water content (RWC) of leaves was measured as an indicator of leaf hydration status as described by Hafez et al. (2020). The second leaf of each plant was extracted, and fresh weight (FW) was determined. Then, the leaves were placed in a beaker with distilled water for 5 h and the saturated weight (SW) was measured. Finally, the leaves were dried at 80 °C to constant weight was attained and dry weight (DW) was determined. RWC was calculated using the formula: RWC (%) = [(FW-DW) / (SW -DW)] x 100. In other words, RWC is a ratio of the amount of water in the leaf tissue at sampling to that present when fully turgid.
-Determination of total chlorophyll content, root viability, proline content and lipid peroxidation
After 20 days of growth in salinity, five plants per treatment were harvested and used for all biochemical determinations.
Total chlorophyll (TChl) content in leaves was determined according to Lichtenthaler and Wellburn (1983). Leaf fresh segments (100 mg) (FW) were cut into small pieces and homogenized in a mortar using 20 mL (V) of 80% (v/v) acetone. The extract was then centrifuged at 8,000xg for 10 min. The supernatant solution was transferred to a colorimeter tube and the optical density or absorbance (A) was measured at 646.8 and 663.2 nm against an 80% acetone blank using a spectrophotometer (Shimadzu UV-1800, Japan). Total chlorophyll content was estimated according to the following formula: TChl (mg g-1 FW) = (7.15 A 663.2 + 18.71 A 646.8) x (V/FW).
Root viability was estimated by measuring the dehydrogenase enzyme activity by using the 2,3,5- triphenyl tetrazolium chloride (TTC) reduction technique (Lopez Del Egido et al., 2017). A sample of 500 mg of roots was cut into small pieces and placed in test tubes containing 5 mL of 0.4% TTC and 5 mL of phosphate buffer (pH = 7) and incubated during 3 h at 37 °C. Then, the samples were extracted into ethyl acetate for 15 min. Absorbance was measured at 485 nm. Results were expressed as absorbance related to root dry weight (A485 g-1 DW).
Determination of free proline content was done according to Bates et al., (1973). Leaves samples (300 mg of fresh weight) were homogenized in 10 mL of 3% aqueous sulfosalicylic acid solution and homogenate filtered through filter paper (Whatman No. 1). Then, 2 mL of filtrate was reacted with 2 mL of 0.2% acid ninhydrin and 2 mL of concentrated glacial acetic acid in a test tube for 1 h at 100 °C. Reaction was then stopped by using ice bath for 15 min. The mixture was extracted with 4 mL of toluene for 20 s with vortex. The absorption of the upper phase was measured by a spectrophotometer (Shimadzu UV-1800, Japan) at a wavelength of 520 nm. Proline concentration was determined using calibration curve and expressed as μmol proline g-1 FW.
Lipid peroxidation was determined by measuring malondialdehyde (MDA) formation using the methodology described by Heath and Packer (1968). For MDA extraction, 300 mg of fresh leaves were homogenized with 3 mL of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 3,000 xg for 10 min. After centrifugation, 1 mL of the supernatant was mixed with 1 mL of the reagent TCA-BHT-TBA (TCA 20%, thiobarbituric acid (TBA) 0.37% and butyl hydroxyl toluene (BHT) 0.01 g), and incubated in hot water (95 °C) for 25 min. The reaction was terminated on ice and the sample was centrifuged at 10,000 xg for 10 min. The absorbance of supernatant was measured at 532 and 600 nm.
-Peroxidase and catalase activity
Peroxidase activity (POX) was determined as described by Cavalcanti et al. (2004) with minimal modifications. A fresh leaf sample of 60 mg (FW) was ground in a mortar, resuspended with 1.5 mL of 10 mM phosphate buffer (pH = 6) and centrifuged at 12,000 xg for 20 min. The supernatant was used for the subsequent enzyme assays. To 100 μL of the extract was added 100 μL of 10 mM sodium phosphate buffer (pH 6), 20 μL of guaiacol (0.25% v/v) and 50 μL of 0.88 M H2O2. POX activity was expressed as reduction in absorbance at 470 nm, measured at 0.5, 1.0, 1.5, and 2.0 min, and related to FW (Cavalcanti et al., 2004).
Catalase activity (CAT) was assayed by measuring the initial rate of disappearance of H2O2 (Dai et al., 2020). An aliquot of 50 μL of the supernatant was extracted and 2000 μL of 50 mM sodium and potassium phosphate buffer (pH = 7) were added, and the reaction was started with 20 μL of 0.88 M H2O2. Enzyme activity was determined by monitoring the decrease in absorbance due to H2O2 decomposition for 1.0 min at 240 nm and related to FW.
-Anatomical study
At 20 days, five plants per treatment in which the best response to salt stress was observed, were randomly selected for the anatomical determinations. All samples were fixed in FAA solution (10 mL formalin + 5 mL glacial acetic acid + 50 mL ethyl alcohol 95% + 35 mL distilled water). The central portion of the second leaf, the middle portion of the main stem and complete roots from the fixed plants were selected. Samples were then embedded in paraffin. Cross sections of 15-20 μm thick were cut, using a rotary microtome (Numak MRF-1, China). Samples were then stained with safranin-Fast Green and mounted in synthetic balm (Rady et al. 2016). Slides were microscopically analyzed (100x), and sections were photographed with a 1.3 MP digital camera (Amscope Md 35) coupled to a binocular optical microscope (Numak XSZ 107 BN, China). By using the image processing software Image J (Schneider et al., 2012), width of mesophyll, length of mesophyll cells, width of adaxial and abaxial epidermis, width of stem bark and vessel elements diameters were measured.
Results
Effect of seed treatment with melatonin on germination in salinity conditions
Germination in salt stress (14 dS m-1) was significantly increased in seeds treated with 10 and 50 µM melatonin solutions when comparing with untreated seeds (MS 0). With a higher concentration (100 µM) a reduction even greater than in untreated seeds was observed. On the other hand, increasing the seed treatment time from 6 to 12 h did not lead to an increase in germination (Fig. 1). With these results, the following experiments with plants were carried out with 6 h of imbibition time.
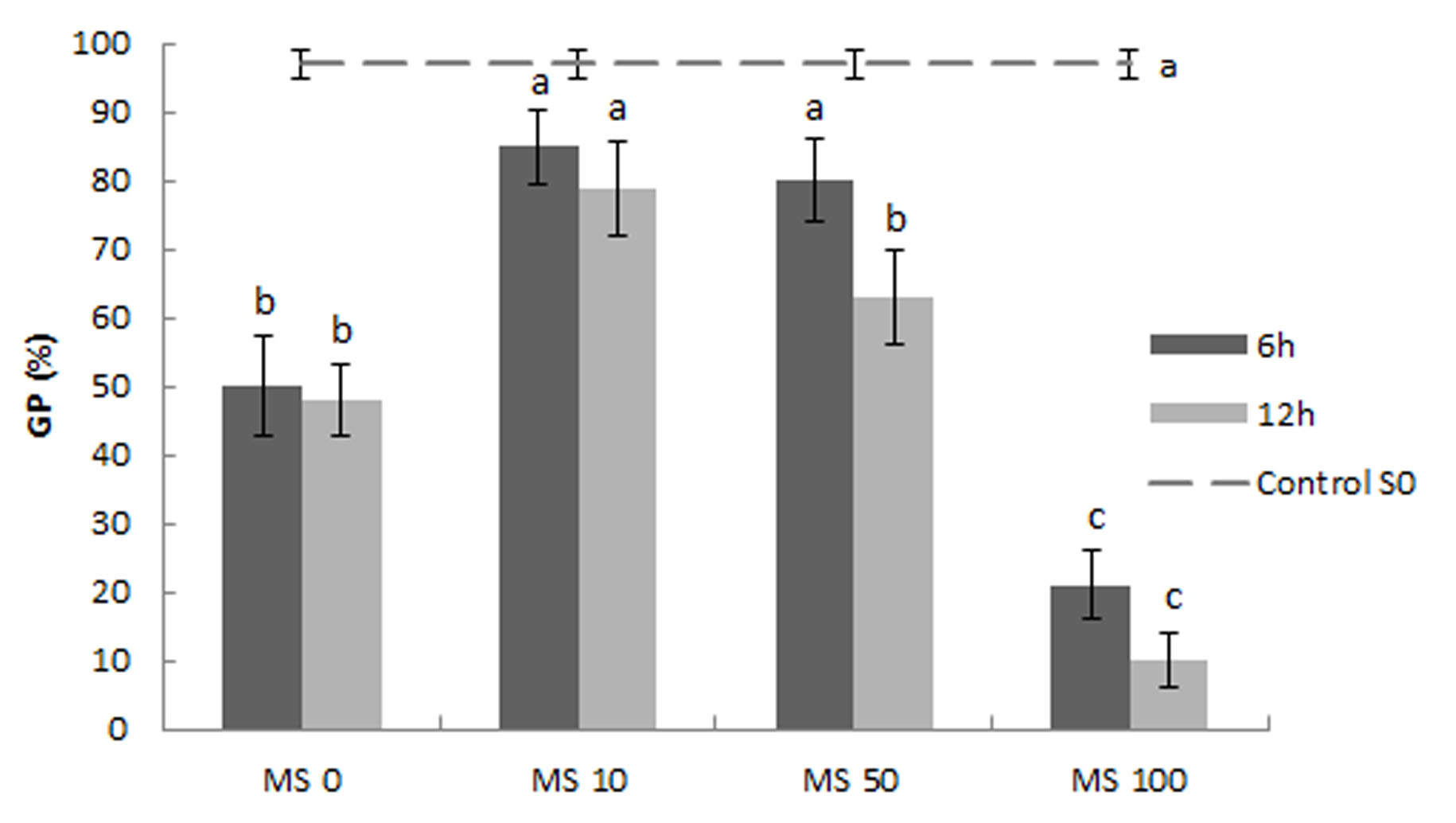
FIGURE 1/FIGURA 1
Figure 1. Effect of seed treatment with melatonin solutions on melon germination percentage (GP) at 14 dS m−1. Vertical bars represent mean ± SE. Different letters indicate a significant difference (P ≤ 0.05) according to Tukey’s test. Figura 1. Efecto del tratamiento de semillas con soluciones de melatonina en el porcentaje de germinación (GP) a 14 dS m-1. Las barras verticales representan la media ± DE. Letras diferentes indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey.
Effect of seed treatment with melatonin on plant growth in salinity conditions
When considering the response in plant growth, in the first place, salt stress caused a reduction of all biometric variables measured (Table 1, Fig. 2).

TABLE 1/TABLA 1
Table 1. Effect of exogenous melatonin on melon growth parameters. Different letters indicate a significant difference (P ≤ 0.05) according to Tukey’s test. Tabla 1. Efecto de la melatonina exógena en los parámetros de crecimiento de melón. Letras diferentes indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey.
Note: Control S0: non-stressed plants, watered with 2.0 dS·m−1 EC solution; MS 0, 10, 50, 100: plants from treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution. Different letters in the same column indicate a significant difference (P ≤ 0.05) according to Tukey’s test
Nota: Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1 CE; MS 0, 10, 50, 100: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1 CE. Letras diferentes en la misma columna indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey
![Figure 2. Phenotypic
appearance of 20 days old melon plants. [A: non-stressed plants, watered with
2.0 dS m−1 EC solution; B, C, D and E: plants from treated seeds
with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with
8.0 dS m−1 EC solution].
Figura 2. Apariencia fenotípica de plantas de melón
de 20 días. [A: plantas no estresadas, regadas con solución con 2,0 dS m-1
CE; B, C, D y E: plantas de semillas tratadas con soluciones de melatonina de
0, 10, 50 y 100 μM,
respectivamente, y regadas con solución con 8,0 dS m-1 CE].](5864885012_gf4.png)
FIGURE 2/FIGURA 2
Figure 2. Phenotypic appearance of 20 days old melon plants. [A: non-stressed plants, watered with 2.0 dS m−1 EC solution; B, C, D and E: plants from treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution]. Figura 2. Apariencia fenotípica de plantas de melón de 20 días. [A: plantas no estresadas, regadas con solución con 2,0 dS m-1 CE; B, C, D y E: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1 CE].
All growth parameters were summarized in the biometric parameter index (BPI), which simplifies visualization of results (Fig. 3). The more negative the values, the greater the negative effect of stress on the biometric variables considered. It is evident that the best phenotypic response, under salt stress conditions, was achieved with 50 µM melatonin solution.
![Figure 3. Effect of seed
treatment with melatonin on Biometric Parameters Index (BPI). [Control S0:
non-stressed plants, watered with 2.0 dS m−1 EC solution; MS 0, 10,
50, 100: plants from treated seeds with 0, 10, 50 and 100 μM melatonin
solutions, respectively, and watered with 8.0 dS m−1 EC solution].
Figura 3. Efecto del tratamiento de semillas con
melatonina en el Índice de Parámetros Biométricos (BPI). [Control S0: plantas
no estresadas, regadas con solución con 2,0 dS m-1 CE; MS 0, 10, 50,
100: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y
100 μM,
respectivamente, y regadas con solución con 8,0 dS m-1 CE].](5864885012_gf5.png)
FIGURE 3/FIGURA 3
Figure 3. Effect of seed treatment with melatonin on Biometric Parameters Index (BPI). [Control S0: non-stressed plants, watered with 2.0 dS m−1 EC solution; MS 0, 10, 50, 100: plants from treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution]. Figura 3. Efecto del tratamiento de semillas con melatonina en el Índice de Parámetros Biométricos (BPI). [Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1 CE; MS 0, 10, 50, 100: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1 CE].
Effect of seed treatment with melatonin on Xylem water potential and relative water in salinity conditions
Evaluation of water status of plants, by measuring xylem water potential (Ψx) (Fig. 4 A) and relative water content (RWC) (Fig. 4 B), indicated a reduction in the values of these parameters because of salt stress. Seed treatment with 50 µM melatonin solution was the most effective treatment to partially alleviating Ψx reduction and completely alleviating RWC reduction.
![Figure 4. Effect of seed
treatment with melatonin on Xylem Water Potential (A) and Relative Water
Content (B) [Control S0: non-stressed plants, watered with 2.0 dS·m−1
EC solution; MS 0, 10, 50, 100: plants from treated seeds with 0, 10, 50 and
100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1
EC solution]. Different letters indicate a significant difference (P ≤ 0.05)
according to Tukey’s test.
Figura 4. Efecto del tratamiento de semillas con
melatonina en el Potencial Hídrico del Xilema (A) y Contenido Relativo de Agua
(B). [Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1
CE; MS 0, 10, 50, 100: plantas de semillas tratadas con soluciones de
melatonina de 0, 10, 50 y 100 μM,
respectivamente, y regadas con solución con 8,0 dS m-1 CE]. Letras
diferentes en la misma columna indican diferencia significativa (P ≤ 0,05) de
acuerdo al test de Tukey.](https://bibliotecavirtual.unl.edu.ar/publicaciones/index.php/FAVEAgrarias/article/download/13318/version/12191/19457/52451/5864885012_gf6.png)
FIGURE 4/FIGURA 4
Figure 4. Effect of seed treatment with melatonin on Xylem Water Potential (A) and Relative Water Content (B) [Control S0: non-stressed plants, watered with 2.0 dS·m−1 EC solution; MS 0, 10, 50, 100: plants from treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution]. Different letters indicate a significant difference (P ≤ 0.05) according to Tukey’s test. Figura 4. Efecto del tratamiento de semillas con melatonina en el Potencial Hídrico del Xilema (A) y Contenido Relativo de Agua (B). [Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1 CE; MS 0, 10, 50, 100: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1 CE]. Letras diferentes en la misma columna indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey.
Effect of seed treatment with melatonin on total chlorophyll, root viability, proline content and malondialdehyde accumulation in salinity conditions
Total chlorophyll content significantly decreased in salt stress (Fig. 5 A). This was mitigated by 50 μM melatonin seed treatment. On the other hand, 10 and 100 μM treatments, did not have a positive effect in preventing chlorophyll degradation.
In roots, 50 μM melatonin concentration significantly increased viability compared to the Control S0 treatment (Fig. 5 B). However, the negative effect of salinity on roots viability, was not completely reversed.
In response to salt stress, plants showed an increase in the contents of proline (Fig. 5 C). In addition, 50 and 100 μM melatonin treatments, further increased the accumulation under salt stress. Meanwhile, the highest increase was measured with 50 μM treatment.
A significant decrease in membrane integrity, estimated by measuring the production of malondialdehyde (MDA), was observed in response to salt stress (Fig. 5 D). Of all the treatments, MS 50 was the one in which the lowest level of MDA was recorded, after the Control S0 treatment.
![Figure 5. Effect of seed
treatment with melatonin on total chlorophyll (A), root viability (B), proline
(C) and malondialdehyde content (D) [Control S0: non-stressed plants, watered
with 2.0 dS·m−1 EC solution; MS 0, 10, 50, 100: plants from treated
seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered
with 8.0 dS m−1 EC solution]. Different letters indicate a
significant difference (P ≤ 0.05) according to Tukey’s test.
Figura 5. Efecto del tratamiento de semillas con
melatonina en Clorofila Total (A), viabilidad de raíces (B), Prolina (C) y
contenido de malondialdehido (D). [Control S0: plantas no estresadas, regadas
con solución con 2,0 dS m-1 CE; MS 0, 10, 50, 100: plantas de
semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0
dS m-1 CE]. Letras diferentes en la misma columna indican diferencia
significativa (P ≤ 0,05) de acuerdo al test de Tukey.](https://bibliotecavirtual.unl.edu.ar/publicaciones/index.php/FAVEAgrarias/article/download/13318/version/12191/19457/52452/5864885012_gf7.png)
FIGURE 5/FIGURA 5
Figure 5. Effect of seed treatment with melatonin on total chlorophyll (A), root viability (B), proline (C) and malondialdehyde content (D) [Control S0: non-stressed plants, watered with 2.0 dS·m−1 EC solution; MS 0, 10, 50, 100: plants from treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution]. Different letters indicate a significant difference (P ≤ 0.05) according to Tukey’s test. Figura 5. Efecto del tratamiento de semillas con melatonina en Clorofila Total (A), viabilidad de raíces (B), Prolina (C) y contenido de malondialdehido (D). [Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1 CE; MS 0, 10, 50, 100: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1 CE]. Letras diferentes en la misma columna indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey.
Effect of seed treatment with melatonin on enzymatic antioxidant defense system in salinity conditions
The activity of peroxidase (POX) and catalase (CAT) enzymes was increased under salt stress. All melatonin treatments significantly improved the activity of these enzymes, although the greatest increase was recorded with 50 μM concentration (Fig. 6 A and B).
![Figure 6. Peroxidase (POX)
(A) and catalase (CAT) (B) activities. [Control S0: non-stressed plants,
watered with 2.0 dS·m−1 EC solution; MS 0, 10, 50, 100: plants from
treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and
watered with 8.0 dS m−1 EC solution]. Different letters indicate a
significant difference (P ≤ 0.05) according to Tukey’s test.
Figura 6. Actividad Peroxidasa (POX) (A) y Catalasa
(CAT) (B). [Control S0: plantas no estresadas, regadas con solución con 2,0 dS
m-1 CE; MS 0, 10, 50, 100: plantas de semillas tratadas con
soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1
CE]. Letras diferentes en la misma columna indican diferencia significativa (P ≤
0,05) de acuerdo al test de Tukey.](https://bibliotecavirtual.unl.edu.ar/publicaciones/index.php/FAVEAgrarias/article/download/13318/version/12191/19457/52454/5864885012_gf8.png)
FIGURE 6/FIGURA 6
Figure 6. Peroxidase (POX) (A) and catalase (CAT) (B) activities. [Control S0: non-stressed plants, watered with 2.0 dS·m−1 EC solution; MS 0, 10, 50, 100: plants from treated seeds with 0, 10, 50 and 100 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution]. Different letters indicate a significant difference (P ≤ 0.05) according to Tukey’s test. Figura 6. Actividad Peroxidasa (POX) (A) y Catalasa (CAT) (B). [Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1 CE; MS 0, 10, 50, 100: plantas de semillas tratadas con soluciones de melatonina de 0, 10, 50 y 100 μM, respectivamente, y regadas con solución con 8,0 dS m-1 CE]. Letras diferentes en la misma columna indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey.
Effect of seed treatment with melatonin on anatomical characteristics
Since the best positive response in previous parameters analyzed was recorded in MS 50 treatment, the anatomical study was performed in this treatment and compared with plants from untreated seeds and in salt stress (MS 0) and non-stressed plants (Control S0) (Fig. 7). No significant differences were observed between treatments when comparing dimensions of different anatomical structures studied (Table 2). In addition, no differences were observed between plants in salinity (MS 0) and those growing without salt stress (Control S0), nor in those plants derived from seeds treated with μM melatonin solutions, treatment in which, in general, the best response was observed.
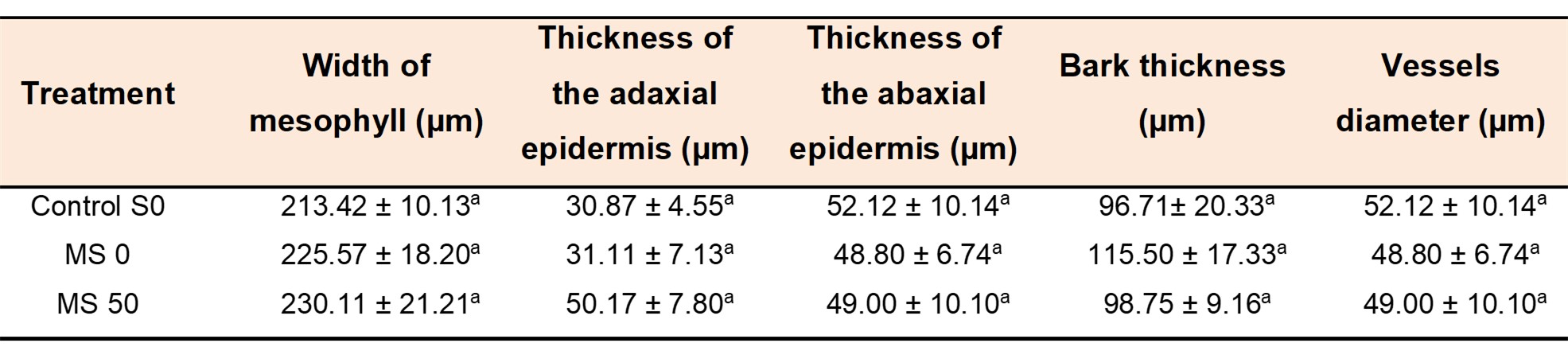
TABLE 2/TABLA 2
Table 2. Structural analysis of leaves and stems sections of melon plants 20 days old Tabla 2. Análisis estructural de hojas y tallos de melón de 20 días
Note: Control S0: non-stressed plants, watered with 2.0 dSm−1 EC solution; MS 0, 50: plants from treated seeds with 0 and 50 μM melatonin solutions, respectively, and watered with 8.0 dS m−1 EC solution. Different letters indicate a significant difference (P ≤ 0.05) according to Tukey’s test.
Nota: Control S0: plantas no estresadas, regadas con solución con 2,0 dS m-1; MS 0 y 50: plantas de semillas tratadas con soluciones de melatonina de 0 y 50 μM respectivamente, y regadas con solución con 8,0 dS m-1 CE. Letras diferentes en la misma columna indican diferencia significativa (P ≤ 0,05) de acuerdo al test de Tukey.
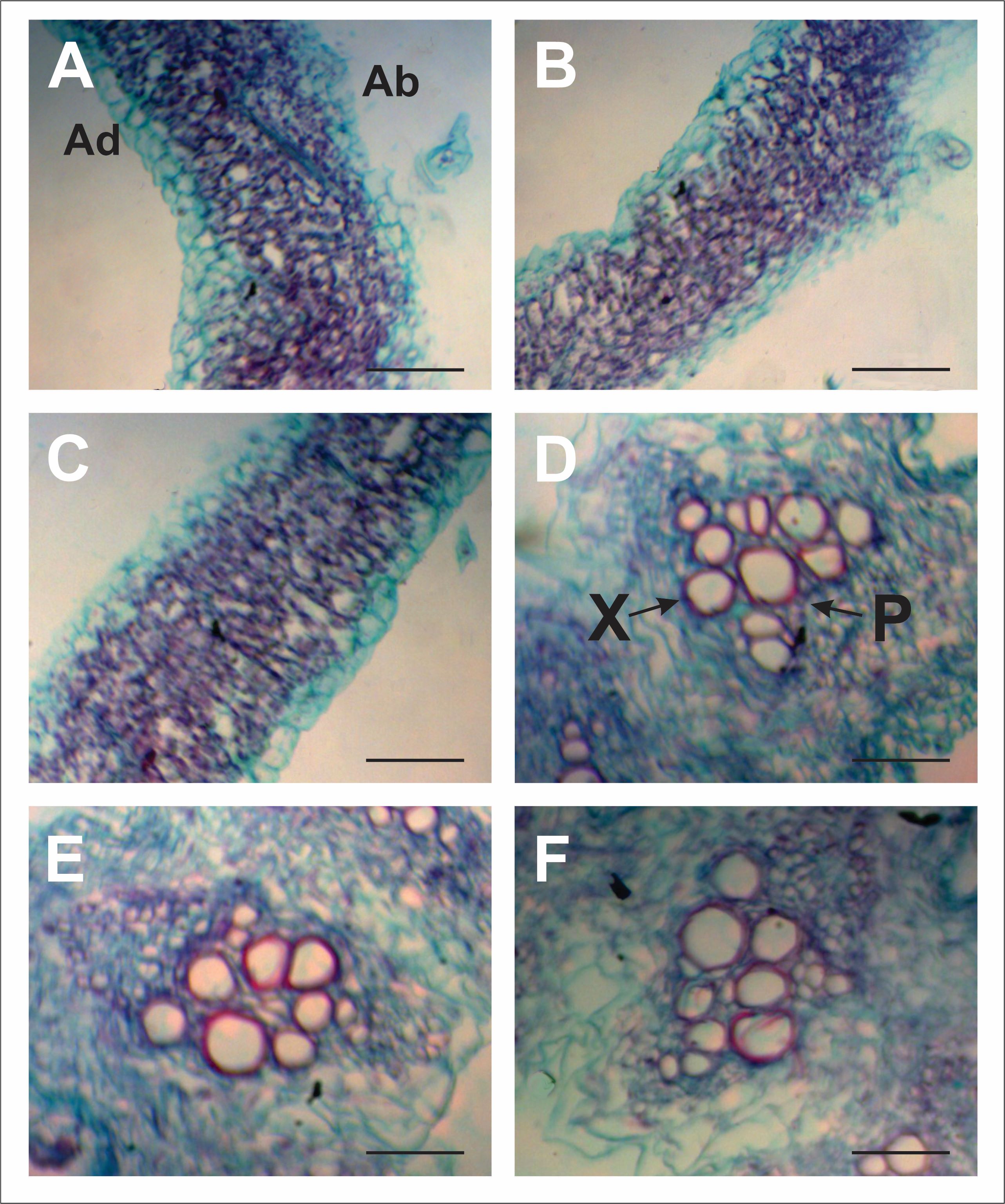
FIGURE 7/FIGURA 7
Figure 7. Cross section of melon leaves of Control S0 (A), MS0 (B) and M50 (C) treatments, and stems of Control S0 (D), MS0 (E) and M50 (F) treatments. References: X: Xylem, P: Phloem, Ad: Abaxial Epidermis; Ab: Abaxial Epidermis. Scale bars: 100 µm Figura 7. Sección transversal de hojas de melón de tratamientos Control S0 (A), MS0 (B) y M50 (C) y tallos de tratamientos Control S0 (D), MS0 (E) y M50 (F). Referencias: X: Xilema, P: Floema, Ad: Epidermis Adaxial; Ab; Epidermis Abaxial. Barras de escala: 100 µm
Discussion
It has been widely reported that salinity is detrimental at all stages of plants cycle, causing significant production losses worldwide (Parihar et al. 2015; Zörb et al. 2019). Germination of seeds as well as their transition to seedlings represent perhaps the most critical stages of a plant’s life cycle. High salinity affects germination due to a combination of osmotic, ionic and oxidative stress (Ibrahim, 2016). Poor seed germination can negatively affect crop establishment and uniformity.
Effect of salt stress on melon seed germination was alleviated by treatment with 10 and 50 μM melatonin solutions (Fig. 1). It has been reported the effect of exogenous melatonin in improving germination under other types of stress, such as drought (Bai et al., 2020; Khan et al., 2019), chilling (Kołodziejczyk et al., 2016; Korkmaz et al., 2021) heat (Hernández et al., 2015; Yu et al., 2022) and contaminated soil stress (Lei et al., 2021). At a higher concentration (100 μM) salinity stress could not be reversed and even had a negative effect, since it resulted in a lower percentage of germination than the Control S0 treatment. This inhibitory effect on germination at higher concentrations of melatonin could be explained by a toxicity effect (Xiao et al. 2019; Wei et al. 2015).
When evaluating the effect of melatonin on plant growth in salt stress, EC value of 8.0 dS m-1 was used considering that crop yield is reduced by approximately 50% (Tanji & Kielen, 2002; Tedeschi et al., 2011). Salinity affected growth of melon plants, as can be seen in Table 1 and Figure 3. These results agree with those reported by Ibrarullah et al. (2019) and Hniličková et al. (2019), who evaluated the growth reduction in different melon cultivars irrigated with NaCl solutions. As Figure 3 shows, plants from melatonin treated seeds with 50 μM exhibited the greatest increase in tolerance to salinity. In corn (Zea mays L.), Erdal (2019) found that spraying plants with melatonin solutions improved growth by maintaining coordination between gene expression and the activity of key enzymes involved in carbon and nitrogen metabolism. In rice (Oryza sativa L.), melatonin spraying increased growth in salt stress by improving the activity the activities of antioxidant enzymes (Wei et al. 2022).
When melatonin concentration was increased (100 μM) a reduction in growth was registered. This response, positive at relatively low concentrations and inhibitory at higher concentrations, was also observed by other researchers (Chen et al. 2009; Wei et al. 2015; Zhang et al. 2014), and a toxicity effect has been suggested as an explanation. Given the multiplicity of reported functions and applications, melatonin has been proposed as a new plant hormone or universal growth regulator (Arnao and Hernández-Ruiz 2019; Kołodziejczyk and Posmyk 2016). Plant hormones and growth regulators fulfill their functions at very low concentrations (Kumudini & Patil, 2019). A similar effect was measured in bean (Phaseolus vulgaris L.) (Azizi et al. 2022), rice (Oryza sativa L.) (L. Wei et al., 2022) and pistachio (Pistacia vera L.) (Kamiab, 2020) with a beneficial effect at relatively low concentrations (10-100 µM), while higher concentrations (>100 µM) had a detrimental effect.
The lower reduction of Ψx in plants from melatonin treated seeds, allows to infer the existence of osmotic adjustment mechanisms, like the accumulation of compatible osmolytes, which are molecules with the ability to reduce the osmotic potential of cells, and assure certain continuity in water absorption, and protect the structure of proteins and enzymes under abiotic stresses (Fedotova, 2019). Proline is the osmolyte most accumulated in plants under osmotic stress (Khanna-Chopra et al., 2019). Under salinity stress represents a higher tolerance in plants due its osmotic adjustment capacity (Khanna-Chopra et al., 2019) and antioxidant activity (Per et al., 2017). In our experiments, stressed plants increased proline content (Fig. 5 C), although in plants from 50 µM melatonin treated seeds the highest content was recorded. This can be related to the decreased degradation of proline by direct antioxidant action of melatonin (Zhang and Zhang 2014) and increased activity of antioxidant enzymes induced by this molecule (Altaf et al., 2022). Siddiqui et al., (2019) also measured an increase in proline content in tomato (Solanum lycopersicum L.) seedlings watered with NaCl and previously treated melatonin solutions. In rice (Oryza sativa L.), Dawood and El-Awadi (2015) applied melatonin to plants growing in cold stress and also registered an increase in proline content.
A significant reduction in total chlorophyll content (TChl) was determined in salt stressed plants (Fig. 5 A). Ibrarullah et al. (2019b) studied the effect of different levels of salinity on the chlorophyll content in eight melon genotypes and in all cases a reduction in the content of this pigment with increasing salinity was detected. This reduction is considered as an indicator of oxidative stress (Luna et al., 2000) and can be explained by the inhibition of chlorophyll synthesis and the activation of its degradation by the enzyme chlorophyllase (Taïbi et al., 2016). Of all treatments, 50 µM was the one that expressed the best result in significantly increasing the chlorophyll level compared to plants from untreated seeds. The increase in TChl content in treated plants has been attributed to the antioxidant properties of melatonin (Altaf et al. 2022; Arnao and Hernández Ruiz 2009). In soybean (Glycine max L.) plants from melatonin-treated seeds had similar chlorophyll content as those untreated plants under normal conditions (Wei et al. 2015). Altaf et al. (2022), growing tomato (Solanum lycopersicum L.) in drought stress, measured an increase in chlorophyll after application of melatonin to plants.
Roots are the organs that represent the main mechanism by which plants supply themselves with water and nutrients. Inhibition of shoot and root development is the primary response of plants to hydric and salt stress (Arif et al., 2020), which will then affect the overall development of the plant. Results indicated a reduction in root viability caused by high salinity (Fig. 5 B), which could be partially reversed only in the 50 µM treatment. Similarly, Altaf et al. (2022) reported an increase in root activity of tomato (Solanum lycopersicum L.) seedlings watered with melatonin solutions and cultivated in drought stress, which was reflected in an ameliorative effect of melatonin in such type of stress. A similar effect was reported by Han et al. (2017) in rice (Oryza sativa L.) cultivated in cold stress.
ROS accumulation in cells is one of the main events that occur in plants subjected to some type of stress (Ahmad et al., 2021). This can lead to an oxidative damage in membranes affecting its structure (Choudhury et al., 2017). The reduction in MDA levels observed in our experiments (Fig. 5 D) indicates a decrease in membrane damage, related to a lower levels of ROS (Ding et al. 2017). This agrees with what was observed in wheat (Triticum aestivum L.) (Cui et al., 2017), maize (Zea mays L.) (Ahmad et al., 2021; Fleta‐Soriano et al., 2017) and bean (Phaseolus vulgaris L.) (Taïbi et al., 2016) growing under abiotic stress. Hydrogen peroxide (H.O.), is the most important and stable of all ROS (Mansoor et al., 2022). In response to biotic and/or abiotic stress an increase in H.O. production usually occurs in plants (Sofo et al., 2015). Among the antioxidant enzymes, peroxidases (POX) and catalases (CAT) are very efficient in degrading H.O. (Anjum et al., 2016). POX enzyme has been proposed as an indicator of the degree of stress in plants (Salah et al., 2015). As can be seen in figures 6 A and B, plants increased POX and CAT enzymes activity in response to salt stress. However, plants from melatonin treated seeds had an even greater increase. Of all treatments, the highest increase in enzyme activity was recorded at 50 μM. Similar results were reported by Wu et al. (2019) who applied foliar melatonin and Ca2+ to melon plants growing in salinity and measured an increase in the activity of antioxidant enzymes with the consequent better growth in salt stress. Zhang et al. (2017) also measured and increase in antioxidant enzyme activity in melon plants treated with melatonin and growing under suboptimal temperatures. In soybean (Glycine max L.) foliar and irrigation application of melatonin led to higher activity of antioxidant enzymes which allowed a better plant performance under drought conditions (Imran et al., 2021). In rice (Oryza sativa L.) melatonin treatment of seeds allowed a better growth of plants under cold stress (Han et al., 2017). This reduction of oxidative damage is fundamentally due to the increase in activity of antioxidant enzymes (Zhan et al., 2019) and direct neutralization of H.O. (Oloumi, 2022).
The absence of anatomical variations in leaves and stems of treated plants (Table 2; Fig. 7) allows us to conclude that the increase in salt tolerance in treated plants is fundamentally due to biochemical processes occurred in the plants. There are no previous reports including anatomical studies in plants treated with melatonin. Shafiee et al. (2019) cultivated different melon varieties in salt stress (8 dS m-1) and, although registered variations at the biochemical level (chlorophyll, proline and antioxidant enzymes), the only anatomical change detected was an increase in the thickness of abaxial epidermis in some varieties.
Conclusion
The results show that melatonin seed treatment induced biochemical and physiological changes in melon plants that allowed an increase in salt stress. Clearly, there is a dose-dependent response. Under the experimental conditions, concentrations of 10 and 50 µM turned were the optimal ones to improve germination. When considering the growth of plants, the best response was observed with of 50 µM melatonin concentration.
In this research, studies at biochemical and anatomical level make it possible to clarify the main mechanisms that allow the increase in salt stress tolerance. Also, this work provides information that, added to the existing knowledge in other crops, brings us closer to the confirmation of the potential benefit that melatonin application represents to improve the performance of crops against different types of stress.
Further studies using advanced molecular techniques would be necessary to unequivocally understand the main mechanisms involved in the increased tolerance to saline stress derived from the application of melatonin.
References
Ahmad, S., Muhammad, I., Wang, G. Y., Zeeshan, M., Yang, L., Ali, I., & Zhou, X. B. (2021). Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biology, 21(1), 1–14.
Altaf, M. A., Shahid, R., Ren, M.-X., Naz, S., Altaf, M. M., Khan, L. U., Tiwari, R. K., Lal, M. K., Shahid, M. A., & Kumar, R. (2022). Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants, 11(2), 309.
Anjum, N. A., Sharma, P., Gill, S. S., Hasanuzzaman, M., Khan, E. A., Kachhap, K., Mohamed, A. A., Thangavel, P., Devi, G. D., & Vasudhevan, P. (2016). Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Environmental Science and Pollution Research, 23(19), 19002–19029.
Arif, Y., Singh, P., Siddiqui, H., Bajguz, A., & Hayat, S. (2020). Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiology and Biochemistry, 156, 64–77.
Arnao, M. B., & Hernández-Ruiz, J. (2019). Melatonin: a new plant hormone and/or a plant master regulator? Trends in Plant Science, 24(1), 38–48.
Arnao, M. B., & Hernández-Ruiz, J. (2020). Is phytomelatonin a new plant hormone? Agronomy, 10(1), 95.
Arnao, M. B., & Hernández Ruiz, J. (2009). Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. Journal of Pineal Research, 46(1), 58–63.
Asociation, I. S. T. (2022). International Rules for Seed Testing. In International Rules for Seed Testing.
Azizi, F., Amiri, H., & Ismaili, A. (2022). Melatonin improves salinity stress tolerance of Phaseolus vulgaris L. cv. Pak by changing antioxidant enzymes and photosynthetic parameters. Acta Physiologiae Plantarum, 44(4), 40.
Bai, Y., Xiao, S., Zhang, Z., Zhang, Y., Sun, H., Zhang, K., Wang, X., Bai, Z., Li, C., & Liu, L. (2020). Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ, 8, e9450.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207. https://doi.org/10.1007/BF00018060
Castañares, J. L., & Bouzo, C. A. (2019). Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Horticultural Plant Journal, 5(2), 79–87.
Cavalcanti, F. R., Oliveira, J. T. A., Martins‐Miranda, A. S., Viégas, R. A., & Silveira, J. A. G. (2004). Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt‐stressed cowpea leaves. New Phytologist, 163(3), 563–571.
Chen, Q., Qi, W. bo, Reiter, R. J., Wei, W., & Wang, B. min. (2009). Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. Journal of Plant Physiology, 166(3), 324–328. https://doi.org/10.1016/j.jplph.2008.06.002
Choudhury, F. K., Rivero, R. M., Blumwald, E., & Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. The Plant Journal, 90(5), 856–867.
Corwin, D. L. (2021). Climate change impacts on soil salinity in agricultural areas. European Journal of Soil Science, 72(2), 842–862.
Cui, G., Zhao, X., Liu, S., Sun, F., Zhang, C., & Xi, Y. (2017). Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiology and Biochemistry, 118, 138–149.
Dai, L., Li, J., Harmens, H., Zheng, X., & Zhang, C. (2020). Melatonin enhances drought resistance by regulating leaf stomatal behaviour, root growth and catalase activity in two contrasting rapeseed (Brassica napus L.) genotypes. Plant Physiology and Biochemistry, 149, 86–95.
Dawood, M. G., & El-Awadi, M. E. (2015). Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colombiana, 20(2), 223–235.
Debnath, B., Islam, W., Li, M., Sun, Y., Lu, X., Mitra, S., Hussain, M., Liu, S., & Qiu, D. (2019). Melatonin mediates enhancement of stress tolerance in plants. International Journal of Molecular Sciences, 20(5), 1040.
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., González, L., Tablada, M., & Robledo, y C. W. (2011). InfoStat versión 2011. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL Http://Www. Infostat. Com. Ar, 8, 195–199.
Ding, F., Liu, B., & Zhang, S. (2017). Exogenous melatonin ameliorates cold-induced damage in tomato plants. Scientia Horticulturae, 219, 264–271.
Dubbels, R., Reiter, R. J., Klenke, E., Goebel, A., Schnakenberg, E., Ehlers, C., Schiwara, H. W., & Schloot, W. (1995). Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography‐mass spectrometry. Journal of Pineal Research, 18(1), 28–31.
Eisa, E. A., Honfi, P., Tilly-Mándy, A., & Mirmazloum, I. (2023). Exogenous Melatonin Application Induced Morpho-Physiological and Biochemical Regulations Conferring Salt Tolerance in Ranunculus asiaticus L. Horticulturae, 9(2), 228.
Erdal, S. (2019). Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Reports, 38(8), 1001–1012.
Fan, J., Xie, Y., Zhang, Z., & Chen, L. (2018). Melatonin: a multifunctional factor in plants. International Journal of Molecular Sciences, 19(5), 1528.
FAOSTAT. (2023). Crops. Crops. https://www.fao.org/faostat/es/#data/QCL
Fedotova, M. V. (2019). Compatible osmolytes-bioprotectants: is there a common link between their hydration and their protective action under abiotic stresses? Journal of Molecular Liquids, 292, 111339.
Fleta‐Soriano, E., Díaz, L., Bonet, E., & Munné‐Bosch, S. (2017). Melatonin may exert a protective role against drought stress in maize. Journal of Agronomy and Crop Science, 203(4), 286–294.
Geilfus, C.-M. (2018). Chloride: from nutrient to toxicant. Plant and Cell Physiology, 59(5), 877–886.
Gopalakrishnan, V., Burdman, S., Jurkevitch, E., & Helman, Y. (2022). From the lab to the field: combined application of plant-growth-promoting bacteria for mitigation of salinity stress in melon plants. Agronomy, 12(2), 408.
Hafez, Y., Attia, K., Alamery, S., Ghazy, A., Al-Doss, A., Ibrahim, E., Rashwan, E., El-Maghraby, L., Awad, A., & Abdelaal, K. (2020). Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy, 10(5), 630.
Hailu, B., & Mehari, H. (2021). Impacts of soil salinity/sodicity on soil-water relations and plant growth in dry land areas: a review. J. Natural Sci. Res, 12(3), 1–10.
Han, Q.-H., Huang, B., Ding, C.-B., Zhang, Z.-W., Chen, Y.-E., Hu, C., Zhou, L.-J., Huang, Y., Liao, J.-Q., Yuan, S., & Yuan, M. (2017). Effects of Melatonin on Anti-oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Frontiers in Plant Science, 8(May), 1–14. https://doi.org/10.3389/fpls.2017.00785
Hasanuzzaman, M., Raihan, M. R. H., Masud, A. A. C., Rahman, K., Nowroz, F., Rahman, M., Nahar, K., & Fujita, M. (2021). Regulation of reactive oxygen species and antioxidant defense in plants under salinity. International Journal of Molecular Sciences, 22(17), 9326.
Hattori, A., Migitaka, H., Iigo, M., Itoh, M., Yamamoto, K., Ohtani-Kaneko, R., Hara, M., Suzuki, T., & Reiter, R. J. (1995). Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochemistry and Molecular Biology International, 35(3), 627–634.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
Hernández, I. G., Gomez, F. J. V., Cerutti, S., Arana, M. V., & Silva, M. F. (2015). Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiology and Biochemistry, 94, 191–196. https://doi.org/10.1016/j.plaphy.2015.06.011
Hniličková, H., Hnilička, F., Orsák, M., & Hejnák, V. (2019). Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant, Soil and Environment, 65(2), 90–96.
Hoagland, D. R., & Arnon, D. I. (1950). The Water-Culture Method for Growing Plants without Soil. California Agricultural Experiment Station, 51, 914–916.
Huang, C. H., Zong, L., Buonanno, M., Xue, X., Wang, T., & Tedeschi, A. (2012). Impact of saline water irrigation on yield and quality of melon (Cucumis melo cv. Huanghemi) in northwest China. European Journal of Agronomy, 43, 68–76.
Hussain, S., Shaukat, M., Ashraf, M., Zhu, C., Jin, Q., & Zhang, J. (2019). Salinity stress in arid and semi-arid climates: Effects and management in field crops. Climate Change and Agriculture, 13.
Ibrahim, E. A. (2016). Seed priming to alleviate salinity stress in germinating seeds. Journal of Plant Physiology, 192, 38–46. https://doi.org/10.1016/j.jplph.2015.12.011
Ibrarullah, H. U. R., Jilani, M. S., Gurmani, A. R., & Ullah, K. (2019a). Toleranca Response of muskmelon genotypes against salinity. Pak. J. Agri. Sci, 56(1), 63–70.
Ibrarullah, H. U. R., Jilani, M. S., Gurmani, A. R., & Ullah, K. (2019b). Tolerance response of muskmelon genotypes against salinity. Pak. J. Agri. Sci, 56(1), 63–70.
Imran, M., Latif Khan, A., Shahzad, R., Aaqil Khan, M., Bilal, S., Khan, A., Kang, S.-M., & Lee, I.-J. (2021). Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants, 13(4), plab026.
Jin, X., Liu, T., Xu, J., Gao, Z., & Hu, X. (2019). Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biology, 19(1), 48.
Kamiab, F. (2020). Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. Journal of Plant Nutrition, 43(10), 1468–1484.
Khan, M. N., Zhang, J., Luo, T., Liu, J., Rizwan, M., Fahad, S., Xu, Z., & Hu, L. (2019). Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Industrial Crops and Products, 140, 111597.
Khanna-Chopra, R., Semwal, V. K., Lakra, N., & Pareek, A. (2019). 5 Proline–A Key Regulator Conferring Plant Tolerance to Salinity and Drought. In Plant Tolerance to Environmental Stress: Role of Phyto Protectants.
Kołodziejczyk, I., Dzitko, K., Szewczyk, R., & Posmyk, M. M. (2016). Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. Journal of Plant Physiology, 193, 47–56. https://doi.org/10.1016/j.jplph.2016.01.012
Kołodziejczyk, I., & Posmyk, M. M. (2016). Melatonin-a new plant biostimulator? Journal of Elementology, 21(4), 1187–1198.
Korkmaz, A., DEĞER, Ö., Szafrańska, K., KÖKLÜ, Ş., KARACA, A., YAKUPOĞLU, G., & Kocacinar, F. (2021). Melatonin effects in enhancing chilling stress tolerance of pepper. Scientia Horticulturae, 289, 110434.
Kumudini, B. S., & Patil, S. V. (2019). Role of plant hormones in improving photosynthesis. Photosynthesis, Productivity and Environmental Stress, 215–240.
Larraburu, E. E., & Llorente, B. E. (2015). Azospirillum brasilense enhances in vitro rhizogenesis of Handroanthus impetiginosus (pink lapacho) in different culture media. Annals of Forest Science, 72(2), 219–229.
Lei, K., Sun, S., Zhong, K., Li, S., Hu, H., Sun, C., Zheng, Q., Tian, Z., Dai, T., & Sun, J. (2021). Seed soaking with melatonin promotes seed germination under chromium stress via enhancing reserve mobilization and antioxidant metabolism in wheat. Ecotoxicology and Environmental Safety, 220, 112241.
Lerner, A. B., Case, J. D., Takahashi, Y., Lee, T. H., & Mori, W. (1958). Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. Journal of the American Chemical Society, 80(10), 2587.
Lichtenthaler, H. K., & Wellburn, A. R. (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions, 11(5), 591–592.
Liu, J., Shabala, S., Zhang, J., Ma, G., Chen, D., Shabala, L., Zeng, F., Chen, Z., Zhou, M., & Venkataraman, G. (2020). Melatonin improves rice salinity stress tolerance by NADPH oxidase‐dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant, Cell & Environment, 43(11), 2591–2605.
Lopez Del Egido, L., Navarro-Miró, D., Martinez-Heredia, V., Toorop, P. E., & Iannetta, P. P. M. (2017). A spectrophotometric assay for robust viability testing of seed batches using 2, 3, 5-triphenyl tetrazolium chloride: using Hordeum vulgare L. as a model. Frontiers in Plant Science, 8, 747.
Luna, C., Garcia‐Seffino, L., Arias, C., & Taleisnik, E. (2000). Oxidative stress indicators as selection tools for salt tolerance. Plant Breeding, 119(4), 341–345.
Mansoor, S., Ali Wani, O., Lone, J. K., Manhas, S., Kour, N., Alam, P., Ahmad, A., & Ahmad, P. (2022). Reactive oxygen species in plants: from source to sink. Antioxidants, 11(2), 225.
Miller, R. O. (1997). Determination of dry matter content of plant tissue: gravimetric moisture. In Handbook of reference methods for plant analysis (pp. 64–65). CRC Press.
Nawaz, M. A., Huang, Y., Bie, Z., Ahmed, W., Reiter, R. J., Niu, M., & Hameed, S. (2016). Melatonin: current status and future perspectives in plant science. Frontiers in Plant Science, 6, 1230.
Oliveira, C. E. da S., Steiner, F., Zuffo, A. M., Zoz, T., Alves, C. Z., & Aguiar, V. C. B. de. (2019). Seed priming improves the germination and growth rate of melon seedlings under saline stress. Ciência Rural, 49.
Oloumi, H. (2022). Melatonin; Growth regulator and strong antioxidant in plants. Journal of Plant Process and Function, 1, 37–54.
Pandey, A. K., Ghosh, A., Rai, K., Fatima, A., Agrawal, M., & Agrawal, S. B. (2019). Abiotic Stress in Plants: A General Outline. In Approaches for Enhancing Abiotic Stress Tolerance in Plants (pp. 1–46). CRC Press.
Parihar, P., Singh, S., Singh, R., Singh, V. P., & Prasad, S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: a review. Environmental Science and Pollution Research, 22(6), 4056–4075.
Per, T. S., Khan, N. A., Reddy, P. S., Masood, A., Hasanuzzaman, M., Khan, M. I. R., & Anjum, N. A. (2017). Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiology and Biochemistry, 115, 126–140.
Pinheiro, D. T., Delazari, F., Nick, C., Mattiello, E. M., Cunha, D., & dos Santos Dias, F. (2019). Emergence and vegetative development of melon in function of the soil salinity. Aust J Crop Sci, 13, 458–464.
Posmyk, M. M., Bałabusta, M., Wieczorek, M., Sliwinska, E., & Janas, K. M. (2009). Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. Journal of Pineal Research, 46(2), 214–223. https://doi.org/10.1111/j.1600-079X.2008.00652.x
Rady, M. M., Taha, R. S., & Mahdi, A. H. A. (2016). Proline enhances growth, productivity and anatomy of two varieties of Lupinus termis L. grown under salt stress. South African Journal of Botany, 102, 221–227.
Safdar, H., Amin, A., Shafiq, Y., Ali, A., Yasin, R., Shoukat, A., Hussan, M. U., & Sarwar, M. I. (2019). A review: Impact of salinity on plant growth. Nat. Sci, 17(1), 34–40.
Salah, S. M., Yajing, G., Dongdong, C., Jie, L., Aamir, N., Qijuan, H., Weimin, H., Mingyu, N., & Jin, H. (2015). Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Scientific Reports, 5, 1–14.
Sarabi, B., Bolandnazar, S., Ghaderi, N., & Ghashghaie, J. (2017). Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: prospects for selection of salt tolerant landraces. Plant Physiology and Biochemistry, 119, 294–311.
Schneider, C. A., Rasband, W. S., & Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9, 671–675. https://doi.org/10.1038/nmeth.2089
Shabala, S., & Munns, R. (2017). Salinity stress: physiological constraints and adaptative mechanism. In S. Shabala (Ed.), Plant stress physiology (2nd ed., pp. 24–63). CAB International.
Shabala, S., Shabala, S., Cuin, T. A., Pang, J., Percey, W., Chen, Z., Conn, S., Eing, C., & Wegner, L. H. (2010). Xylem ionic relations and salinity tolerance in barley. The Plant Journal, 61(5), 839–853.
Shafiee, H., Haghighi, M., Farhadi, A., & Ehteman, M. (2019). The effect of salinity on physiological, biochemical and anatomical characteristics of different varieties of melon. Journal of Plant Process and Function, 8(33), 325–338.
Shalaby, O. A. E.-S., & El-Messairy, M. M. (2018). Humic acid and boron treatment to mitigate salt stress on the melon plant. Acta Agriculturae Slovenica, 111(2), 349–356.
Sharif, R., Xie, C., Zhang, H., Arnao, M. B., Ali, M., Ali, Q., Muhammad, I., Shalmani, A., Nawaz, M. A., & Chen, P. (2018). Melatonin and its effects on plant systems. Molecules, 23(9), 2352.
Siddiqui, M. H., Alamri, S., Al-Khaishany, M. Y., Khan, M. N., Al-Amri, A., Ali, H. M., Alaraidh, I. A., & Alsahli, A. A. (2019). Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. International Journal of Molecular Sciences, 20(2), 353.
Singh, S., & Bharati, L. K. (2016). Cultivation and bioprospecting of perennial cucurbits. In M. Pessarakli (Ed.), Handbook of cucurbits. Growth,cultural practices and physiology (pp. 95–108). CRC.
Sofo, A., Scopa, A., Nuzzaci, M., & Vitti, A. (2015). Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. International Journal of Molecular Sciences, 16(6), 13561–13578.
Suárez-Hernández, Á. M., Vázquez-Angulo, J. C., Grimaldo-Juárez, O., Duran, C. C., González-Mendoza, D., Bazante-González, I., & Mendoza-Gómez, A. (2019). Production and quality of grafted watermelon in saline soil. Horticultura Brasileira, 37(2), 215–220.
Taïbi, K., Taïbi, F., Abderrahim, L. A., Ennajah, A., Belkhodja, M., & Mulet, J. M. (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African Journal of Botany, 105, 306–312.
Tanji, K. K., & Kielen, N. C. (2002). Agricultural drainage water management in arid and semi-arid areas. FAO.
Tedeschi, A., Lavini, A., Riccardi, M., Pulvento, C., & d’Andria, R. (2011). Melon crops (Cucumis melo L., cv. Tendral) grown in a mediterranean environment under saline-sodic conditions: Part I. Yield and quality. Agricultural Water Management, 98(9), 1329–1338.
Ulas, F., Aydın, A., Ulas, A., & Yetisir, H. (2019). Grafting for sustainable growth performance of melon (Cucumis melo) under salt stressed hydroponic condition. European Journal of Sustainable Development, 8(1), 201–210.
Wei, J., Li, D., Zhang, J., Shan, C., Rengel, Z., Song, Z., & Chen, Q. (2018). Phytomelatonin receptor PMTR 1‐mediated signaling regulates stomatal closure in Arabidopsis thaliana. Journal of Pineal Research, 65(2), e12500.
Wei, L., Zhao, H., Wang, B., Wu, X., Lan, R., Huang, X., Chen, B., Chen, G., Jiang, C., & Wang, J. (2022). Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. Journal of Plant Growth Regulation, 41(6), 2108–2121.
Wei, W., Li, Q. T., Chu, Y. N., Reiter, R. J., Yu, X. M., Zhu, D. H., Zhang, W. K., Ma, B., Lin, Q., Zhang, J. S., & Chen, S. Y. (2015). Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. Journal of Experimental Botany, 66(3), 695–707. https://doi.org/10.1093/jxb/eru392
Wu, X., Ren, J., Huang, X., Zheng, X., Tian, Y., Shi, L., Dong, P., & Li, Z. (2021). Melatonin: Biosynthesis, content, and function in horticultural plants and potential application. Scientia Horticulturae, 288, 110392.
Wu, Y., Gao, Q., Huang, S., & Jia, S. (2019). Enhancing salt tolerance in melon by exogenous application of melatonin and Ca2+. Pak. J. Bot, 51(3), 781–787.
Xiao, S., Liu, L., Wang, H., Li, D., Bai, Z., Zhang, Y., Sun, H., Zhang, K., & Li, C. (2019). Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PloS One, 14(6), e0216575.
Yasuor, H., Yermiyahu, U., & Ben-Gal, A. (2020). Consequences of irrigation and fertigation of vegetable crops with variable quality water: Israel as a case study. Agricultural Water Management, 242, 106362.
Yu, Y., Deng, L., Zhou, L., Chen, G., & Wang, Y. (2022). Exogenous melatonin activates antioxidant systems to increase the ability of Rice seeds to germinate under high temperature conditions. Plants, 11(7), 886.
Zhan, H., Nie, X., Zhang, T., Li, S., Wang, X., Du, X., Tong, W., & Song, W. (2019). Melatonin: A small molecule but important for salt stress tolerance in plants. International Journal of Molecular Sciences, 20(3), 709.
Zhang, H. J., Zhang, N., Yang, R. C., Wang, L., Sun, Q. Q., Li, D. B., Cao, Y. Y., Weeda, S., Zhao, B., Ren, S., & Guo, Y. D. (2014). Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA<inf>4</inf> interaction in cucumber (Cucumis sativus L.). Journal of Pineal Research, 57(3), 269–279. https://doi.org/10.1111/jpi.12167
Zhang, H., & Zhang, Y. (2014). Melatonin: a well‐documented antioxidant with conditional pro‐oxidant actions. Journal of Pineal Research, 57(2), 131–146.
Zhang, Y. P., Xu, S., Yang, S. J., & Chen, Y. Y. (2017). Melatonin alleviates cold-induced oxidative damage by regulation of ascorbate–glutathione and proline metabolism in melon seedlings (Cucumis melo L.). The Journal of Horticultural Science and Biotechnology, 92(3), 313–324.
Zörb, C., Geilfus, C., & Dietz, K. (2019). Salinity and crop yield. Plant Biology, 21, 31–38.
Additional information
Acknowledgements: The authors are grateful to Lic. Débora Mas, from Experimental Station
INTA AMBA, for her assistance in the edition of figures.
Declarations Competing Interest:: The authors declare they have no conflicts of interest.


