Case Report
Infiltrating mammary carcinoma in a cat with a congenital disorder: prognostic interest of histological examination
Carcinoma mamario infiltrante en una gata con un trastorno congénito: interés pronóstico del examen histológico
Infiltrating mammary carcinoma in a cat with a congenital disorder: prognostic interest of histological examination
FAVE Sección Ciencias Veterinarias, vol. 21, e0006, 2022
Universidad Nacional del Litoral

Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional.
Recepción: 06 Abril 2022
Aprobación: 27 Junio 2022
Resumen: En esta publicación se describe el manejo y la evolución clínica de un carcinoma mamario felino (CMF) maligno en una gata mestiza de ocho años de edad con un trastorno congénito. La eficacia de la extirpación de la masa tumoral y el pronóstico del caso se establecieron mediante el examen histológico de rutina postoperatorio. Tras una mastectomía completa se extirpó un bulto de 6 cm de diámetro en la glándula mamaria torácica izquierda de aspecto multinodular y exudado purulento de coloración rojiza. El análisis histopatológico mostró la presencia de numerosos émbolos tumorales linfovasculares y células con alta frecuencia de figuras mitóticas con núcleos hipercromáticos. Estos hallazgos fueron consistentes con una neoplasia agresiva de mal pronóstico, lo que explicaba el fallecimiento de la paciente a las 7 semanas de la cirugía. Los hallazgos histológicos y citológicos son de importancia crítica para los clínicos en el manejo de las neoplasias mamarias más comunes en los gatos, con el fin de aliviar de la mejor manera posible a los animales con cánceres que amenazan su vida. También podría servir como modelo de cáncer para avanzar en el conocimiento de patologías similares en otras especies.
Palabras clave: felino, tumor mamario, embolia linfática, pronóstico.
Abstract: This report describes the handling and clinical evolution of a malignant feline mammary carcinoma (FMC) in an eight-year-old female crossbred cat with a congenital disorder. Hence, the efficacy of tumor mass removal and the case prognosis was established by postoperative routine histological examination. A 6 cm diameter lump in the left thoracic mammary gland of multinodular appearance and purulent exudate with a reddish hue was excised after the entire mastectomy. The histopathological analysis showed the presence of numerous lymphovascular tumor emboli and frequently mitotic cells with hyperchromatic nuclei. These were consistent with an aggressive malignancy with a poor prognosis explaining the patient's death within 7 weeks after surgery. Histology and cytology findings are of critical importance to clinicians in the management of the most common mammary malignancies in cats, to provide the best possible relief to animals with life-threatening cancer. It could also serve as a cancer model for advancing knowledge in congenitally affected humans.
Keywords: feline, mammary tumor, lymphatic emboli, prognosis.
Introduction
Mammary carcinoma is one of the most common tumors in cats. In Algeria, the prevalence of feline mammary cancer and its histological classes is not documented. In the UK, squamous cell carcinoma (11.1 %) is the second most common cancer in this species after lymphoma (14 %) (Rodríguez et al. 2021). These feline tumors are almost always malignant neoplasms with a high rate of cell growth and metastasis (Govoni et al. 2021). The lack of appropriate veterinary health infrastructure limits the prospects for tumor surveillance in companion animals (Rodríguez et al. 2021).
Tumor size and early recognition of its stage by cytohistological analysis remain essential for the diagnosis, treatment and prognosis of mammary cancers (Morris 2013). Due to the similarities of cytohistopathological findings in human and feline mammary gland tumors, the latter has been considered useful as an animal model of human breast cancer (Nascimento & Ferreira, 2021).
In this context, this report aims to highlight the importance of the anatomopathological examination of the surgical specimen in the diagnosis and prognosis of mammary tumors in a cat with a congenital disorder as a possible model allowing advances in knowledge of similar affections in humans.
Case presentation
The case concerns an 8-year-old crossbred cat that was born in July 2013 with a congenital total absence of the right forelimb. The cat was not spayed and had two breeding seasons per year. She gave birth to her first kittens at 8 months (March 2014) and the last ones at 8 years in June 2021 with 5 kittens per litter (all alive) and nursed them normally.
The cat, in the last month of her last pregnancy (May 2021), was presented to a private veterinary clinic for consultation of a two-month-old coalescing ulcerated mammary mass of the left thoracic location (Figure 1.A) displaying fluid exudation (blood and pus). It was decided to perform surgical removal of this tumor mass after parturition. Before the operation, amoxicillin 250 mg in oral suspension and a cod liver oil ointment were prescribed twice a day.
The regional mastectomy was performed (August 2021) by a simple "melon rib" surgical technique used for large nodular lesions (Figure 1.B-E). To do this, general anesthesia was performed using an intra-muscularly injection of Tiletamine-Zolazepam combination (10 mg/kg). The mass was completely excised (Figure 1.F) and the large blood vessels were ligated. The axillary lymph node was not removed because, in addition to the operative difficulty, the Veterinary Surgical Oncology Society only recommends it in cases of hypertrophy or positive cytology (Morris 2013). The reconstruction was carried out in two planes, the first plane concerned the muscular walls and the second was the closure of the skin and subcutaneous tissue. The pain management was ensured by subcutaneous injection of 0.25 mg/kg of flunixin-meglumine (50 mg/ml). As a postoperative antibiotherapy, 0.5 ml of Dihydrostreptomycin; Benzylpenicillin Procaine (intramuscular, 300,000 IU/ml) was administered. In addition, amoxicillin 250 mg in oral suspension (15 mg/Kg) was prescribed twice a day for over a week.

Figure 1. Patient and surgical removal of mammary tumor. A. Ulcerated carcinoma (empty arrow), B-E. Process of mastectomy, E. Cat with a congenital absence of the right forelimb (empty arrowhead), F. Excised mass (black arrow).
For histopathological analysis, the excised mass was weighed and measured along the three axes. It was then inked for subsequent microscopic evaluation of the excision limits (margin). Next, oriented specimens were taken, prepared, and processed to be stained with routine hematoxylin and eosin (H&E) for microscopic examination.
Results
The surgical procedure allowed the removal of an operatory piece covered with skin, weighing 46 g and 6 cm x 6 cm x 3.5 cm (Figure 2.A). On cross-section, the tumor occupied the entire specimen. It has a whitish multinodular appearance in contact with the deep plane (Figure 2.B).

Figure 2. Gross appearance of the excised mass. A. Operatory piece, B. Cross-section of specimen.
Microscopic examination on multiple oriented specimens and serial sections reveals an infiltrating carcinomatous proliferation (Figure 3.A) that ulcerates the skin covering and extends to the deep plane whereas the striated muscle sampled was healthy (Figure 3.B). This proliferation is made up of glandular formations and cribriform masses bordered by Cubo-cylindrical cells with basophilic cytoplasm and hyperchromatic nuclei, somewhat irregular and frequently mitotic. The architecture of the tumor is papillary, trabecular and tubular (Figure 3.C&D) with a fibrous stroma. Numerous lymph vessel emboli were present (Figure 3.E&F). Fibrinoleukocyte exudate (Figure 3.G), ulceration stigmata and tumor necrosis (Figure 3.H) were also observed. The ink used is in contact with the excision limit indicating a positive margin. This latter is therefore incomplete meaning to tumor recurrence.
After the operation, the patient's owners went on vacation with the operated cat for 15 days. During this period and until the next veterinary control, 3 weeks after the operation, the postoperative course was uneventful. Later, 51 days after the operation (October 2021, the owners informed the cat dead after a deterioration of its condition (loss of appetite, weakness, and weight loss) 3 weeks ago and after suffering from respiratory distress in the last days of its life.
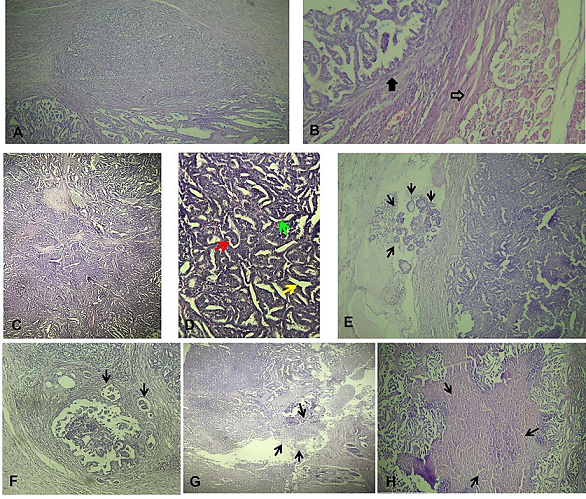
Figure 3. Microscopic aspect of the tumor using hematoxylin and eosin (H&E). A. Tumor with various architecture (X40), B. Carcinoma infiltration (black arrow) and the sampled healthy striated muscle (empty arrow, X100), C & D. Papillary (red arrow), trabecular (green arrow) and tubular (yellow arrow) architecture of tumor (high magnification), E & F. Lymphovascular tumor emboli (arrows, X200) G. Fibrinoleukocyte exudate (arrows), H. Tumor necrosis (arrows, X40).
También se puede descargar imagen en mayor resolución desde: https://figshare.com/articles/figure/FAVE_Ciencias_Veterinarias_Suplemental_Matterials_Hadef_et_al_Infiltrating_mammary_carcinoma_in_a_cat_with_a_congenital_disorder_prognostic_interest_of_histological_examination/20286588
Discussion and conclusions
The present case of mammary carcinoma concerns an older cat with a congenital absence of the right anterior limb. These formation abnormalities have been associated in humans with the hereditary susceptibility to different types of cancers, including those of the breast due to the breast cancer susceptibility gene BRCA1 mutation (Kwiatkowski et al. 2020). This association is to be hypothesized in the cat considering the recent findings supporting similarities between feline and human mammary carcinomas, especially in terms of mutations in exon 9 of BRCA1 (Govoni et al. 2021).
The results obtained from the histopathological examination as well as the clinical evolution of the cat's condition until her death, show that the excised tumor corresponds to an infiltrating and ulcerative metastatic FMC with papillary, trabecular and tubular architecture.
The presence of lymphatic emboli means the presence of tumor cell clusters in the vessels and that its dissemination is already done, the lymphatic pathway is the privileged swarming pathway for mammary tumors in the cat (Raharison et al. 2009) leading to regional lymph node and lung metastases (Goldschmidt et al. 2011; Losco 1986; Soares et al. 2021). This could justify the deterioration of the patient's state of health with the appearance of respiratory distress until his death 7 weeks after surgery expressing the metastatic potential of the tumor and the prognosis, in this case, is more pejorative.
The tumor size (T) represents along with cancer spread to adjacent lymph nodes (N) and distant metastasis (M) the three criteria used in the TNM clinical grading system (Morris 2013). The results of the macroscopic pathological examination show that the surgical specimen had a size that exceeded the tumor size 3 (T3) score of 3 cm described by Ito et al. (1996) according to the World Health Organization (WHO) classification. Such a score (T3) allows the tumor to be classified as at least stage III according to the WHO clinical staging system.
Lymphatic embolization is a strong prognostic factor related to the risk of metastatic generalization and cancer-related death (Besnard 2016). Given the prognostic value of lymphatic embolization, we based on histological grading instead of a clinical TNM grading of feline mammary carcinoma following Besnard's (2016) suggestion that a histological grading is preferred in which lymph node stage would be replaced, if unknown as in the present case, by the presence of lymph emboli.
Using the modified system corresponding to the histological classification of mammary carcinoma in cats established by Preziosi et al. (2002), it seems that the removed tumor is in stage II since the carcinoma is invasive with the presence of emboli and/or lymph node metastasis.
The skin ulceration noted on clinical examination of the patient could be an interesting prognostic criterion if the system proposed for grading breast cancer (BC) by the American Joint Committee on Cancer (Edge et al. 2010) is taken into consideration. This system considers BC with skin ulceration (SU) regarding erosion as pT4b and which was associated with a poorer prognosis in patients with tumor sizes larger than 2 cm to 5 cm.
Regarding the operation efficacy, microscopic examination revealed the presence of cancer cells at the very edge of the cut tissue that may be hard for the surgeon to see, which is considered a positive margin indicating a higher risk that the tumor will recur in the same location after the intervention. This is in line with the postoperative clinical course compatible with that of a high malignancy grade according to the WHO with a survival time relatively close to the median survival time of 1 month for clinical stage IV reported by Ito et al. (1996). It seems that the period between mastectomy and death due to mammary carcinoma (specific survival) changes depending on several prognostic factors applicable to systems gradation as noted by Dagher et al. (2019). In agreement with these authors, in this case, lymphovascular invasion proves to be a powerful prognostic factor for the practicing veterinarian in feline mammary carcinomas, as it has been recommended to be used independently of any grading system (Dagher et al. 2019), especially when clinicopathologic data are not fully available. Similarly, in human, lymphovascular invasion was identified as an independent predictor of both breast cancer-specific survival and distant metastasis-free survival in patients with operable breast cancer (Rakha et al. 2012) and for overall survival in patients with lymph node-positive patients with primary invasive breast cancer (Song et al. 2011). In comparison, from an observational cohort study of 229 cases of canine mammary tumors, tumor diameter was found to be the main predictor of local recurrence/distant metastasis and an independent prognostic indicator of survival whereas lymphatic invasion and histologic grade were limited predictive of survival (Rasotto et al. 2017).
This report describes in a cat with a congenital disorder the postoperative outcome of one of the fatal forms of common malignant mammary tumors which may be a model for human breast cancer. In similar cases, far from histological classification purpose, Lymphatic emboli by cancer cells with skins ulceration seem to be useful prognosis indicators when associated with clinical symptoms. Furthermore, the use of histological examination to predict surgeon treatment efficiency in cases of dominant malignant tumors should be promoted and more applied by the vets to improve pets’ welfare regarding cancer pain management.
Materiales suplementarios
References
Besnard F. 2016. Emboles lymphatiques des carcinomes mammaires invasifs félins: amélioration de leur détection par immunohistochimie et valeur pronostique. Bull. Acad. Vet. Fr. 169: 176-185.
Dagher E, Abadie J, Loussouarn D, Campone M, Nguyen F. 2019. Feline invasive mammary carcinomas: Prognostic value of histological grading. Vet. Pathol. 56: 660-670.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds.). 2010. AJCC cancer staging manual. Ed. Springer Inc., New York. 718 pp.
Goldschmidt M, Peña L, Rasotto R, Zappulli V. 2011. Classification and grading of canine mammary tumors. Vet. Pathol. 48: 117-131.
Govoni VM, Da Silva TC, Guerra JM, Pereira IVA, Queiroga FL, Cogliati B. 2021. Genetic variants of BRCA1 and BRCA2 genes in cats with mammary gland carcinoma. Vet. Comp. Oncol. 19: 404-408.
Ito T, Kadosawa T, Mochizuki M, Matsunaga S, Nishimura R, Sasaki N. 1996. Prognosis of malignant mammary tumor in 53 cats. J. Vet. Med. Sci. 58: 723-726.
Kwiatkowski F, Perthus I, Uhrhammer N, Francannet C, Arbre M, Bidet Y, 2020. Association between hereditary predisposition to common cancers and congenital multimalformations. Congenit. Anom. (Kyoto) 60: 22-31.
Losco PE. 1986. Local and peripheral eosinophilia in a dog with anaplastic mammary carcinoma. Vet. Pathol. 23: 536-538.
Morris J. 2013. Mammary tumours in the cat: Size matters, so early intervention saves lives. J. Feline Med. Surg. 15: 391-400.
Nascimento C, Ferreira F. 2021. Tumor microenvironment of human breast cancer, and feline mammary carcinoma as a potential study model. Biochim. Biophys. Acta (BBA) - Reviews on Cancer 1876: 188587.
Preziosi R, Sarli G, Benazzi C, Mandrioli L, Marcato PS. 2002. Multiparametric survival analysis of histological stage and proliferative activity in feline mammary carcinomas. Res. Vet. Sci. 73: 53-60.
Raharison F, Mogicato G, Sautet J. 2009. The lymphatic system of mammary glands in female cat. Block dissection surgical technique in the removal mammary tumors. Rev. Méd. Vét. 160: 562-568.
Rakha EA, Martin S, Lee AHS, Morgan D, Pharoah PDP, Hodi Z, MacMillan D, Ellis IO. 2012. The prognostic significance of lymphovascular invasion in invasive breast carcinoma: vascular invasion in breast cancer. Cancer 118: 3670-3680.
Rasotto R, Berlato D, Goldschmidt MH, Zappulli V. 2017. Prognostic significance of canine mammary tumor histologic subtypes: An observational cohort study of 229 cases. Vet. Pathol. 54: 571-578.
Rodríguez J, Killick DR, Ressel L, Espinosa de los Monteros A, Santana A, Beck S, Cian F, McKay JS, Noble PJ, Pinchbeck GL, Singleton DA, Radford AD. 2021. A text-mining based analysis of 100,000 tumours affecting dogs and cats in the United Kingdom. Sci. Data. 8: 266.
Soares M, Correia J, Nascimento C, Ferreira F. 2021. Anaplastic mammary carcinoma in cat. Vet. Sci. 8: 77.
Song YJ, Shin SH, Cho JS, Park MH, Yoon JH, Jegal YJ. 2011. The role of lymphovascular invasion as a prognostic factor in patients with lymph node-positive operable invasive breast cancer. J. Breast Cancer 14:198-203.